Hello!
Recently, the heat is settling down “just a little” in Japan!
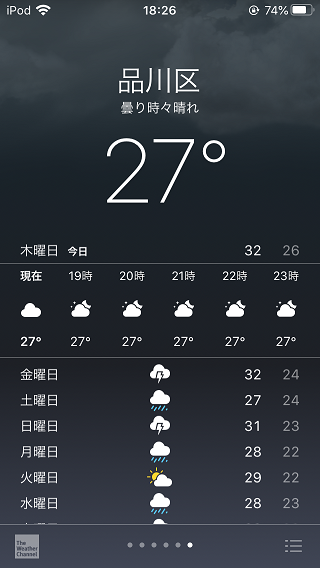
Finally, there are a few days when the maximum temperature drops below 30 degrees Celsius!
Such September is the month when the vaccine for “new coronavirus (COVID-19)” under development by Oxford University in the United Kingdom and AstraZeneca, a major British pharmaceutical company, is scheduled to be completed!
“It’s already September! I’m looking forward about that!”
I was really looking forward to it … but the other day, the news of a “temporary suspension” came in about the clinical trial of the vaccine, so I would like to summarize it this time!
I introduce the “summary article” that tells the daily situation of “new coronavirus (COVID-19) infection” in Tokyo and Japan every day,
but I have reorganized up and updated the latest information about
“the flow so far about vaccine development and therapeutic drugs,” and
“when will we be able to inoculate?”!
The article is here ↓
While organizing this, I was delighted again, saying,
“Is it finally here (September)? It was a long time … but it will be completed in a little while!” …
Meanwhile, a little worrisome news came in.
Here is the information on the “Worrisome news” that I told you in the “Daily Summary Article”! ↓
アストラゼネカ 新型コロナのワクチン 臨床試験 一時的に中断
A new coronavirus (COVID-19) vaccine being developed by British pharmaceutical giants AstraZeneca and Oxford University, but seems to have temporarily suspended clinical trials …
I have introduced this vaccine under development by AstraZeneca and Oxford University many times in this summary article and so on, and this is the fastest vaccine in the world scheduled to be completed in September this month. But…
After reading the article carefully, apparently a clinical trial conducted in the UK confirmed that one vaccinated person had severe symptoms.
It is said that the causal relationship with vaccination is unknown, and it seems that specific symptoms are being investigated.
By the way, this final stage clinical trial (Phase 3 trial), which has been conducted for several weeks, is said to have participated in a total of 30,000 people from the United States, the United Kingdom, Brazil, and South Africa.
It seems that this clinical trial has been conducted in Japan since the end of August.
(By the way, Japan seems to have been temporarily suspended due to this time.)
I would like to summarize some of the reactions of experts.
BBC Fergus Walsh Medical Editor
“It is not uncommon for large-scale clinical trials to be interrupted. Every time a participant is hospitalized for unknown reasons due to poor physical condition, it is interrupted every time.”
Oxford University spokeswoman
“Some people get sick by chance in large-scale clinical trials, but in the case we need to be carefully verified separately from the clinical trial.”
Professor Ken Ishii, Institute of Medical Science, University of Tokyo
“It’s unclear why it stopped, but clinical trials sometimes stop.”
“Vaccines cause an irreversible immune response once they are hit, so there should be no safety issues, so you should watch over each and every thing, not happy or sad.
Ministry of Health, Labor and Welfare in Japan
“It is not uncommon for clinical trials to be interrupted at the final stage.”
“It’s not a discontinuation, it’s just an’interruption’, so I don’t think it will affect Japan’s vaccine security at this point.”
Specially Appointed Professor Tetsuo Nakayama of Kitasato University
“Safety confirmation is indispensable for vaccine development. In the third stage clinical trials, the number of subjects will increase and it is not known what will happen, so it is important to thoroughly verify any undesired reactions.”
Approximately 30,000 people are conducting this final stage clinical trial, and one has symptoms …
I hope it’s not a side effect of the vaccine …
And I hope that one of them who has symptoms is safe because he is also participating in the clinical trial…
In this time when “I’m looking forward to completion soon”, I got some anxious news…
However,
the vaccine being developed by American pharmaceutical giant Pfizer is scheduled to be completed in October, so don’t give up hope and wait for the information after that!
And in connection with that …
Nine companies, including AstraZeneca, a major British pharmaceutical company that develops vaccines for the new coronavirus (COVID-19), and Pfizer, the United States, have jointly announced a declaration that
“we will proceed with the development of safety in the best possible way”!
・ Vaccine safety is our top priority.
・ Always give top priority to the health of the inoculators.
・ After confirming safety and efficacy through three-stage clinical trials, proceed to approval.
It seems to emphasize these.
And in the same article, a survey of about 20,000 people in 27 countries, including Japan, conducted from July to August this year found that 26% of those people were worried about their health effects about vaccination. They said They were reluctant to give vaccination.
I want you to be able to get vaccinated as soon as possible, but safety is also important …
On the other day (September 9th), the news of “Vaccine Development Temporary Suspension” like above ran around the world!
After that, there wasn’t much follow-up news on major news sites in Japan, so I was worried and looked it up, but there was a follow-up report after that!
Some news has included important information in pieces, so I will introduce them together!
アストラゼネカ、ワクチン治験の被験者に脊椎炎症 – ロイター
(* For those who can read Japanese: If you read only the title of the source, you may be a little worried, but it is not such a content, so please pass through the title once! lol)
First of all,
the situation of the person of “Severe symptoms were confirmed in one vaccinated person”,
・ Subject is a British woman
・ Symptom is “transversal spondylitis”, a neuropathy that causes inflammation of the spine.
*According to AstraZeneca’s CEO Pascal Soriot, a woman in the United Kingdom has begun to develop nervous system disorders.
*Also, according to AstraZeneca spokeswoman Michelle Macel, no final diagnosis has been made in this case.
(The diagnosis will not be confirmed until more tests are performed)
It is reported like this.
・ Women are recovering.
・ She may be discharged from the hospital where she was hospitalized within 9 or 10 days.
(↑ Does that mean that the symptoms are not so severe …?)
I had the above information, but I’m really glad that it didn’t seem to be such a serious symptom!
And
・ Actually, “interruption” was the second time (the first time was in July), and the first time was neuropathy, but at that time it was not related to the vaccine due to multiple sclerosis.
・Clinical trials were suspended worldwide due to possible side effects.
・The clinical trial is expected to resume in the first half of next week.
It seems that both the subjects and clinical trials (vaccine development) are okay!
relieved!
I was really relieved!
By the way,
According to Professor Stephen Evans (Pharmaceutical Epidemiology) of the University of London School of Public Health and Tropical Medicine, said that
“Temporary interruption in clinical trials can happen by accident, and each time we have to verify the causal relationship with the vaccine.”
“It’s not uncommon for clinical trials with more than 70 subjects to have this happen,”
and
James Gil, an honorary clinical professor at the University of London School of Medicine, also said that
“It is a model for transparent research that properly discloses information (Even bad news) to the general public who are waiting for news of COVID-19 vaccine development.”
“It is rather good to suspend the clinical trial to verify safety without giving in to the political pressure to “develop and complete it quickly”. “
That was the opinion of the majority of experts in the news!
(Because I think that all people including me are interested in the new coronavirus)
To be honest, this “interruption” report was pretty shocking news for the people.
But when I looked it up, there seemed to be no problem and I was really relieved!
So, while telling this to as many people as possible, I would like to continue to wait for the completion of the new coronavirus (COVID-19) vaccine!
Until then, let’s be careful about infection prevention measures for a while!
As a tool for infection prevention measures ↓
This is a summary of the aftereffects of the “New Coronavirus (COVID-19)” that I summarized the other day.↓
The content is, “If you get infected, you may still suffer from sequelae two years later.”
Please don’t get infected anyway …!
And this is
“How long time the materials such as paper, cloth, plastic, etc. need to eliminating the risk when coronavirus is attached, and how effective disinfection is.”
If you are interested, please take a look!
↑ I will continue to write useful articles like this.
I will continue to do my best to put it together!
Please be very careful when you go out!
AcertainFox512
*This is “COVID-19 Trend Calendar in Tokyo” as of Sep 12↓
◆Changes in New Coronavirus Infection in Tokyo◆
| Aug | |||||||
| Sun | Mon | Tue | Wed | Thu | Fri | Sat | Total |
| – | – | – | – | – | – | 472 | 2187 |
| 292 | 258 | 309 | 263 | 360 | 462 | 429 | 2373 |
| 331 | 197 | 188 | 222 | 206 | 389 | 385 | 1918 |
| 260 | 161 | 207 | 186 | 339 | 258 | 256 | 1667 |
| 212 | 95 | 182 | 236 | 250 | 226 | 247 | 1448 |
| 148 | 100 | – | – | – | – | – | – |
| Aug | |||||||
| Sun | Mon | Tue | Wed | Thu | Fri | Sat | Total |
| – | – | 170 | 141 | 211 | 136 | 181 | 1087 |
| 116 | 77 | 170 | 149 | 276 | 187 | 226 | 1201 |
| – | – | – | – | – | – | – | – |
| – | – | – | – | – | – | – | – |
| – | – | – | – | – | – | – | – |
*Unit: person (number of infected people on that day)



コメント
Keep this going please, great job!
Hey I know this is off topic but I was wondering
if you knew of any widgets I could add to my blog that automatically tweet
my newest twitter updates. I’ve been looking for a plug-in like
this for quite some time and was hoping maybe you would have some experience with something like this.
Please let me know if you run into anything. I truly enjoy reading your blog and I look forward to your new updates.
WOW just what I was looking for. Came here by
searching for here
Feel free to visit my blog post; taruhanprediksi.net
Woah! I’m really digging the template/theme of this website.
It’s simple, yet effective. A lot of times it’s tough to
get that “perfect balance” between usability and visual appearance.
I must say you have done a superb job with this. In addition, the blog loads super quick for me
on Safari. Excellent Blog!
Feel free to surf to my website :: agen poker deposit pulsa
I was pretty pleased to uncover this site. I wanted to
thank you for your time just for this fantastic read!! I
definitely really liked every little bit of it and I have you book marked to see new stuff on your web site.
My web-site; agen s128 sabung ayam
I love your blog.. very nice colors & theme. Did you make this website yourself or
did you hire someone to do it for you? Plz respond as I’m looking to construct
my own blog and would like to know where u got this from.
kudos
Feel free to visit my web-site :: sabung ayam
Great delivery. Great arguments. Keep up the amazing effort.
Also visit my webpage … klikberita.org
What’s Happening i’m new to this, I stumbled upon this I
have found It positively helpful and it has helped me out loads.
I am hoping to contribute & aid different customers like its helped me.
Good job.
Howdy I am so excited I found your website, I really found you by error, while I was looking on Digg for something
else, Anyhow I am here now and would just like to say thanks for a incredible post
and a all round interesting blog (I also love the theme/design), I don’t have time to look over it all at the moment but I have saved it and also added your RSS feeds,
so when I have time I will be back to read much more, Please do keep up the excellent
work.
I’m extremely inspired with your writing skills as smartly as
with the layout to your weblog. Is that this a paid
subject matter or did you customize it yourself?
Anyway keep up the nice quality writing, it’s uncommon to look a nice weblog like this one nowadays..
Thanks for this post, I am a big fan of this web site would like to go along updated.
Feel free to visit my blog … PlayPods Reviews
Wow, this paragraph is good, my sister is analyzing such things, so I am going to inform her.
It’s amazing for me to have a web page, which is useful designed for my experience.
thanks admin
Thanks for every other informative blog. The place else could I get that kind of information written in such
a perfect manner? I’ve a undertaking that I am simply now working on, and I have been at the
glance out for such info.
My homepage – https://domino-qiuqiu.net
Hello! Someone in my Facebook group shared this site with us so I came to look
it over. I’m definitely enjoying the information. I’m book-marking and will be tweeting this to my followers!
Excellent blog and fantastic design and style.
Look at my website: freebetweb.net
I think the admin of this web site is in fact working hard for his web page,
because here every stuff is quality based data.
Hey! Do you use Twitter? I’d like to follow you if
that would be ok. I’m undoubtedly enjoying your blog and look
forward to new posts.
Your style is unique in comparison to other people I have read stuff from.
Thank you for posting when you’ve got the opportunity, Guess I’ll
just book mark this web site.
I like what you guys are up too. This type of clever work and reporting!
Keep up the excellent works guys I’ve added you guys to blogroll.
Every weekend i used to visit this website, as i want enjoyment, as
this this web site conations truly good funny information too.
It’s amazing to visit this website and reading the views of all mates regarding this paragraph, while I am also eager of
getting knowledge.
Having read this I thought it was extremely informative. I appreciate you
spending some time and effort to put this informative article together.
I once again find myself spending a significant amount of time both reading and commenting.
But so what, it was still worth it!
my web page … link s128
What’s up everyone, it’s my first pay a visit at this web page, and piece of writing is
really fruitful designed for me, keep up posting such content.
Check out my web site :: login s128
This post will assist the internet users for creating new blog or even a weblog from start to end.
Take a look at my web blog – s128 sabung ayam
Ahaa, its pleasant discussion concerning this piece
of writing at this place at this web site, I have read all
that, so now me also commenting here.
Thanks for sharing your thoughts about About therapeutic drugs and vaccines.
Regards
my website: poker idn pulsa
Quality content is the secret to invite the viewers to pay a visit the web site, that’s what this web site is providing.
Look into my web site :: login s128
A recent deal with Penn saw them obtain access to seven new states which includes West Virginia
and Pennsylvania.
Hi there, I wish for to subscribe for this webpage to take
newest updates, thus where can i do it please help.
You really make it seem really easy along with your presentation but I find this
matter to be really one thing that I believe I would by no means understand.
It seems too complicated and very wide for me. I’m looking forward for your next publish,
I’ll attempt to get the hang of it!
Take a look at my web blog … Lumo Stat Cream
Simply to follow up on the up-date of this theme on your web-site and would really want
to let you know simply how much I liked the time you took
to write this valuable post. In the post, you spoke regarding how to truly handle this thing with all comfort.
It would be my own pleasure to get some more suggestions from your web page and come as much as offer some others what I discovered from you.
Many thanks for your usual wonderful effort.
Here is my page: Solid Performance System Review
What’s up, yeah this paragraph is truly good and I have learned lot of things from it on the topic of blogging.
thanks.
Here is my web page – Vita Grow XL Reviews
It’s a pity you don’t have a donate button! I’d definitely
donate to this outstanding blog! I suppose for now i’ll settle for book-marking and
adding your RSS feed to my Google account. I look forward to new updates and will talk about
this site with my Facebook group. Chat soon!
Thanks for sharing your thoughts about About therapeutic drugs and vaccines.
Regards
Hello There. I found your blog using msn. This is an extremely well written article.
I’ll be sure to bookmark it and come back
to read more of your useful information. Thanks for the post.
I will certainly comeback.
Hello! This post couldn’t be written any better!
Reading this post reminds me of my good old room mate!
He always kept talking about this. I will forward
this page to him. Fairly certain he will have a good read.
Many thanks for sharing!
Hurrah, that’s what I was looking for, what a material! present here at this website, thanks admin of this website.
It’s truly a great and helpful piece of info. I am happy that you just shared this useful information with us.
Please stay us informed like this. Thank you for sharing.
Take a look at my homepage: Artemis DX8000 Reviews
Note the odds on the Tributes listed on the right side are pretty much copies of the odds of the Tributes
listed on the left side.
Terrific article! That is the type of info that are meant to be shared
around the web. Shame on the seek engines for not positioning this publish upper!
Come on over and discuss with my web site . Thank you =)
I like the valuable info you provide in your articles.
I will bookmark your weblog and check again here regularly.
I’m quite certain I will learn many new stuff right here!
Best of luck for the next!
Would love to always get updated great website!
Also visit my blog :: Steelovil Review
I love your blog.. very nice colors & theme. Did you create this website yourself or did you hire someone to do it for you?
Plz answer back as I’m looking to construct my own blog and
would like to find out where u got this from. kudos
Look into my page: https://agenpokerresmi.com/
Generally I do not read post on blogs, but I would
like to say that this write-up very forced me to take a
look at and do it! Your writing style has been amazed me.
Thank you, quite great article.
Piece of writing writing is also a excitement, if you be acquainted
with after that you can write if not it is complicated to write.
It’s actually a cool and helpful piece of info.
I’m glad that you just shared this useful info with us.
Please stay us informed like this. Thanks for sharing.
Stunning story there. What occurred after? Good luck!
Check out my blog post: https://judipulsa88.xyz/
Hello just wanted to give you a quick heads up.
The words in your post seem to be running off the screen in Ie.
I’m not sure if this is a format issue or something to do
with browser compatibility but I figured I’d post to let you know.
The style and design look great though! Hope
you get the issue resolved soon. Kudos
It’s in fact very complex in this busy life to listen news on Television,
so I simply use world wide web for that reason, and obtain the latest information.
my web site – Beauty Skin Serum
This is very attention-grabbing, You are a very professional blogger.
I’ve joined your rss feed and look forward to in search of extra of your
great post. Additionally, I have shared your website in my social networks
Here is my site … True Libido Boost Review
That is a great tip especially to those new to the blogosphere.
Brief but very precise info… Appreciate your sharing this one.
A must read article!
My web page … s128
If some one needs to be updated with latest technologies therefore he must be
go to see this website and be up to date everyday.
Hey there just wanted to give you a quick heads
up and let you know a few of the pictures aren’t loading correctly.
I’m not sure why but I think its a linking issue.
I’ve tried it in two different web browsers and
both show the same results.
My web site; daftar poker online
Wow, this paragraph is nice, my younger sister is analyzing these things, thus
I am going to convey her.
Yes! Finally someone writes about maroquinerie
de luxe.
Thanks for finally writing about > Details about the suspension of the “Vaccine Expected by
the World” scheduled to be completed in September! [New Coronavirus] [Oxford University]
[AstraZeneca] [COVID-19] | Tokyo ! Japan !
Life now ! < Liked it!
Here is my blog; Vita Grow XL Pills
Wonderful goods from you, man. I’ve understand your stuff previous to and you’re just extremely magnificent.
I actually like what you’ve acquired here, certainly like what you are
stating and the way in which you say it. You make it enjoyable and you still take care of
to keep it sensible. I cant wait to read far more from you.
This is really a great website.
Greetings from Idaho! I’m bored at work so
I decided to check out your blog on my iphone during lunch break.
I love the info you present here and can’t wait
to take a look when I get home. I’m shocked
at how quick your blog loaded on my phone ..
I’m not even using WIFI, just 3G .. Anyhow, awesome site!
I do trust all the ideas you have offered for your post.
They’re very convincing and will definitely work.
Nonetheless, the posts are too brief for beginners.
May just you please lengthen them a bit from next time?
Thank you for the post.
I’m not that much of a internet reader to be honest but your blogs really
nice, keep it up! I’ll go ahead and bookmark your site to come back in the future.
Many thanks
Hi, I do believe this is an excellent web site.
I stumbledupon it 😉 I may revisit yet again since i have saved as a favorite it.
Money and freedom is the greatest way to change, may you be rich and
continue to help other people.
Hello there, I discovered your site by the use of Google even as
searching for a similar subject, your web site came up, it appears great.
I’ve bookmarked it in my google bookmarks.
Review my blog Neuronol Review
Wonderful goods from you, man. I’ve understand your stuff previous
to and you are just extremely great. I actually like what you
have acquired here, really like what you’re stating and the way in which you say it.
You make it enjoyable and you still take care of to keep it wise.
I can’t wait to read far more from you. This is really a wonderful site.
First off I want to say excellent blog! I had a quick question in which I’d like to
ask if you don’t mind. I was interested to find out how you center yourself and clear your head before
writing. I have had a tough time clearing my mind in getting my
ideas out there. I do take pleasure in writing but it just seems like the first 10 to 15 minutes tend to be lost just
trying to figure out how to begin. Any suggestions or hints?
Many thanks!
Wow! After all I got a weblog from where I know how to actually get helpful information regarding my study and knowledge.
Spot on with this write-up, I absolutely feel this site needs a lot
more attention. I’ll probably be returning to read more, thanks for the info!
I’ve been surfing online more than 4 hours today, yet I never found any interesting article like yours.
It’s pretty worth enough for me. In my view, if all website owners and bloggers made good content as you did,
the net will be a lot more useful than ever before.
I always was interested in this topic and stock still am, regards for putting up.
My web blog; XPro Drone
An intriguing discussion is worth comment. I believe that you should write more on this subject, it may not be a taboo subject but typically folks
don’t speak about such topics. To the next!
Many thanks!!
Hey there, You’ve done an incredible job.
I will certainly digg it and personally suggest to my friends.
I’m sure they’ll be benefited from this website.
Attractive section of content. I just stumbled upon your site
and in accession capital to assert that I acquire actually enjoyed account your
blog posts. Anyway I will be subscribing to your
augment and even I achievement you access consistently fast.
Thanks very nice blog!
Do you mind if I quote a couple of your articles as long as
I provide credit and sources back to your weblog? My blog site
is in the exact same niche as yours and my visitors would genuinely benefit from
some of the information you present here. Please let me know if
this ok with you. Thank you!
Here is my webpage … Testoryze Reviews
It is not my first time to visit this website, i am browsing this site dailly and obtain pleasant data
from here all the time.